Electrochemical Biosensor Qualification
Ever since I received one of those wrist worn fitness trackers for Christmas, I’ve been amazed at how sensor technology continues to evolve, especially to improve our health. I’ve been reading many articles and journal papers that have discussed the increasing technological advancements for sensors used in biology/medicine, environmental systems, and bioelectronics which have the potential to change personal healthcare, strengthen security systems, help protect the environment, food, and water supplies, and improve overall our way of life. Most of this is centered on the electrochemical sensor or biosensor. What are these sensors and how are they characterized and validated?
What is an electrochemical sensor?
Electrochemical sensors are used for many applications in diverse industries, including environmental and gas monitoring, medical applications like determining glucose concentration, as well as in the automotive and agricultural industries. Electrochemical sensors come in a wide range of differing designs; they may have two or three electrodes and could be potentiometric, amperometric, or voltammetric. Some sensors are based on organic electronic devices or nano-structures.
Electrochemical biosensors are normally based on enzymatic catalysis of a reaction that produces or consumes electrons (such enzymes are rightly called redox enzymes). The sensor substrate may contain three electrodes; a reference electrode, a working electrode and a counter electrode. The target analyte is involved in the reaction that takes place on the working electrode surface, and the reaction may cause either electron transfer across the double layer (producing a current) or can contribute to the double layer potential (producing a voltage). One can either measure the current (rate of flow of electrons is now proportional to the analyte concentration) at a fixed potential or the potential can be measured at zero current (this gives a logarithmic response).
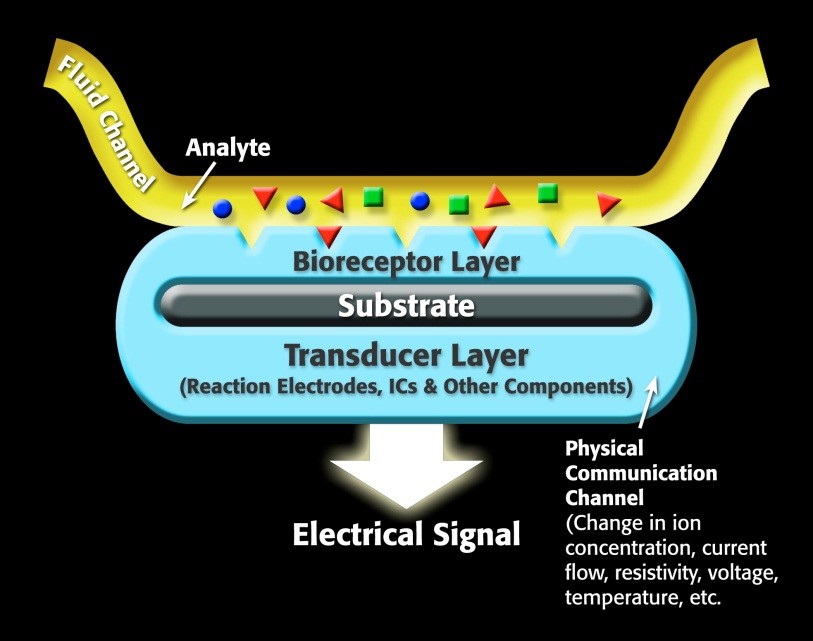
For a specific biosensor, the sensor contains a receptor, which is a molecule that recognizes the target analyte. The transducer portion of the biosensor converts the recognition event into a measurable signal that correlates with the quantity or presence of the chemical or biological target that is of interest. A generalized biosensor model is show in Figure 1.
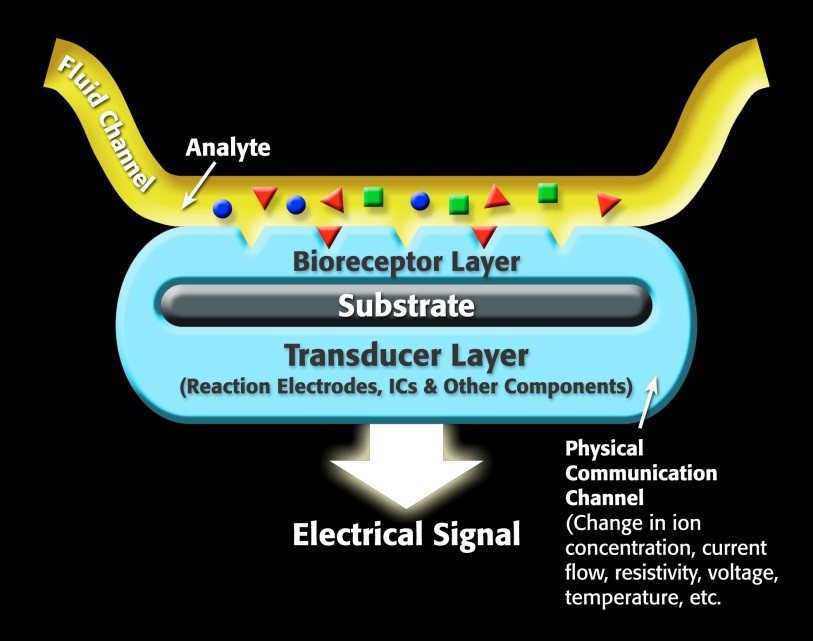
Figure 1. Representation of a generic biosensor.
In another example, the electrochemical potentiometric biosensor, (potential produced at zero current) gives a logarithmic response with a high dynamic range. Such biosensors are often made by screen printing the electrode patterns on a plastic substrate, coated with a conducting polymer and then some protein (enzyme or antibody) is attached. They have only two electrodes and are extremely sensitive and robust.
What test equipment is the best to use to characterize an electrochemical sensor?
Choosing the optimum test equipment is crucial for electrochemical sensor R&D.
Measuring the output of a potentiometric sensor, for example may require a very high impedance voltmeter, with high input impedance (>1014 ohms). In other cases, such as when testing an amperometric gas sensor very sensitive ammeter functionality may be needed. These instrument characteristics can be found in Source Measure Unit (SMU) instruments. Figure 3 shows a simple amperometric gas sensor.
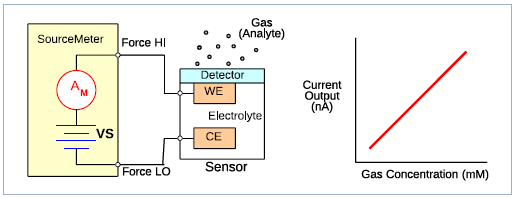
Figure 3. Amperometric sensor characterized using a SourceMeter Source Measure Unit
When a gas comes in contact with the working electrode (WE) a chemical reaction occurs, either oxidation or reduction, depending on the sensor. In an amperometric sensor, current flows between the counter electrode (CE) and the working electrode (WE). The current output, which is related to the gas concentration, is measured by a sensitive ammeter. If necessary, a third electrode, a reference electrode, can be added to the sensor to control a potential.
Are you saying that a Source Measure Unit works just like a potentiostat?
The simple answer is yes! SMUs are very versatile instruments that can source and measure voltage or current. They can compare the results as parameters (e.g. Cyclic Voltammetry) or over time (e.g. Chronoamperometry). Source-measure units, in many cases, can perform as well as potentiostats.
Keithley’s new 2450-EC and 2460-EC Potentiostats operate the very same way as that potentiostat you might be using today. These solutions ease the learning curve for non-traditional users and significantly reduce the struggle to configure measurement functions using cumbersome, multilayer menu structures and confusing multi-function buttons. The 2450-EC and 2460-EC uses an icon-based flat menu system just like that used on smart technology consumer products, like the array of app icons displayed on a tablet or smartphone.

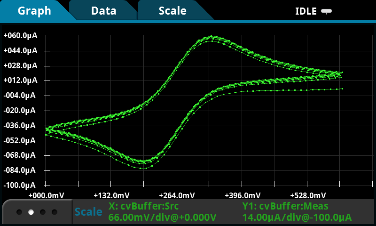
Figure 3. Advanced touchscreen GUI on Keithley’s Model 2450-EC Potentiostat
You can learn more at this related blog: How Source Measure Units can be used as Potentiostats for Cyclic Voltammetry, Chronoamperometry and other Electrochemical Tests
For more information on the products discussed in this blog, click on the following links:
- Keithley 2450-EC and 2460-EC Potentiostats
- Source Measure Units
To learn more about Biosensor characterization, download the applications brief: Biosensor/Transducer Qualification Using the Model 2450 Interactive SourceMeter® SMU Instrument.
John Tucker is a Senior Marketing and Product Manager for the Keithley product line of Tektronix, Inc. He has been with Keithley for over 28 years and has served in a variety of positions, including test engineer, applications engineer, applications manager, product manager, and business development manager. He is currently the product manager for Keithley’s Source Measure Unit (SMU) product line and the new 2450-EC and 2460-EC Potentiostat instruments. He is also a member of the Keithley Electrochemistry team.

 KALİBRASYON LABORATUVARI
KALİBRASYON LABORATUVARI







































































